New Agile sensors for Surface Temperature data collection
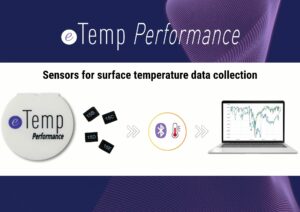
Surface temperature is a key parameter in many scientific and industrial protocols.The eTemp Performance system enables reliable and continuous data collection of this temperature, combining
Find out about our innovative solutions, dedicated to health and wellness key players.
Improve the patient care and facilitate the return home preserving comfort.

Facilitate a continuous and cost effective access to physiological data.

Reduce the risks for high-level athletes and support performance optimization.

BodyCAP develops, industrializes and markets miniature wireless electronic sensors and global monitoring solutions.
Specialized in developing connected devices for physiological monitoring, particularly core body temperature measurement, the company collaborates with top international experts to design highly innovative products. These products anticipate the needs of the most demanding users across various fields:
Certified ISO 13485 and ISO 9001, BodyCAP ensures that its products comply with all applicable standards and regulations. The multidisciplinary development team excels in:
BodyCAP has also demonstrated its capability to industrialize complex medical electronic devices in collaboration with European manufacturers, ensuring maximum reliability and quality.

Surface temperature is a key parameter in many scientific and industrial protocols.The eTemp Performance system enables reliable and continuous data collection of this temperature, combining

eCelsius Performance mentioned in a new scientific publication!🏃♂️ The aim of this study was to evaluate the thermoregulatory responses of recreational runners during an urban

📊 European Aquatics and Loughborough University (UK) have launched a research project to monitor the core body temperature of swimmers in divergent water temperature environments
BodyCAP is committed to developing miniaturized, reliable, and precise physiological monitoring solutions that meet the needs of medical professionals and human physiology researchers.
Founded in 2011 in Caen, Normandy (France), the company emerged from the intersection of human physiology research and microelectronics innovation. Among key physiological parameters, core body temperature stands out as a critical health indicator. However, continuous core body temperature monitoring has traditionally been challenging, requiring invasive devices like rectal or esophageal probes.
At the time, it became clear that advancements in electronic sensor miniaturization and wireless communication technologies were still underutilized in human health monitoring. BodyCAP thus focused on developing innovative miniaturized sensors, designed to simplify and enhance core body temperature monitoring while ensuring a comfortable and non-invasive user experience.
2011: BodyCAP founded
2015: ISO 9001:2015 and ISO 13485:2016 certification
2016: Launch of the first version of the eCelsius Performance system
2021: Launch of the eCelsius Performance Connect system
2023: eCelsius Medical system cleared by the FDA
2024: Launch of the eCelsius Medical system
2025: Launch of the eTemp Performance system
BodyCAP brings together expertise in connected sensor development, industrialization of comprehensive monitoring solutions, commercialization, and customer support, all within a proven quality management system.







Find out more about Bodycap
and its products in video
Documentation related
to all the products
How to buy
our products
BodyCAP, a company that develops innovative solutions, serving actors in the world of health & well-being.
3 rue du Docteur Laennec
14200 Hérouville Saint-Clair
FRANCE
Copyright © 2020 All rights reserved | Conception & realisation : Agile | Legal notice